New Gene Therapy for Inherited Blindness: Donor-supported Research Helped Save Hannah’s Sight
New Gene Therapy for Inherited Blindness: Donor-supported Research Helped Save Hannah’s Sight
The summer before she started second grade, Hannah Reif saw a star for the first time.
“I took her outside and said, ‘Hannah, can you see that little white light in the sky? That’s a star,’” says her mom, Amy. “And she said, ‘I can see it, I can see it!’ She was so happy.”
That moment was a breakthrough for Hannah, 7. Just a few months earlier, she couldn’t see much of anything unless she was in a bright, well-lit room. And she had been preparing for a future in which she wouldn’t be able to see anything at all.

Be a Child’s Hero
Generous donors make breakthrough discoveries like Hannah’s possible. Help us change even more lives for children who are so close to a cure.
A devastating diagnosis: Leber congenital amaurosis
Hannah’s vision problems first became apparent when she was just a few months old. She wasn’t making eye contact, and would only look out windows and at bright lights. Amy and her husband, Chris, also noticed that Hannah’s eyes moved rapidly, a condition called nystagmus. Hannah’s pediatrician referred her to an ophthalmologist, who arranged for her to have a special vision test called an electroretinogram (ERG) at Children’s Hospital of Philadelphia (CHOP).
At CHOP, the Reifs met with ophthalmologist William Anninger, MD, who delivered devastating news: Hannah had Leber congenital amaurosis (LCA), a rare retinal disease. Patients with LCA have very limited vision and eventually — usually in their 20s or 30s — become completely blind.
“He told us, ‘It’s OK to mourn the loss of her vision,’” Amy says. “It always makes us cry when we think about it.”

Genetic testing revealed Hannah had a type of LCA caused by mutations in the RPE65 gene. The news gave the Reifs hope: They had recently learned of a clinical trial underway at CHOP and Penn Medicine testing a gene therapy for patients with LCA caused by RPE65 mutations. At the time, Hannah was too young to enroll in the trial, and no other treatments were available. There was nothing the Reifs could do but wait.
A new gene therapy brings life-changing news
Hannah came to CHOP every year for checkups with the Ophthalmology team, and received physical and occupational therapy. She learned to ride a bike and a scooter. She played with her brothers, Matthew and Jacob. She was able to walk without assistance, but she was learning to use a navigational cane, just in case.
And then, on Dec. 19, 2017, her family received the news they had been waiting for: LUXTURNA, the gene therapy for LCA developed at CHOP and Penn, had just been approved by the Food and Drug Administration. It was the first gene therapy for a genetic disease to be approved in the United States.
CHOP eye surgeon Albert Maguire, MD — who developed the therapy with Jean Bennett, MD, PhD, a professor of Ophthalmology at the University of Pennsylvania, and Katherine High, MD, who was Director of CHOP’s Center for Cellular and Molecular Therapeutics at the time — was one of just a few doctors in the nation to offer it. The Reifs knew and trusted Dr. Maguire, so the decision to have the procedure done at CHOP was an easy one.
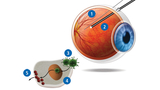
How LUXTURNA works: Using DNA, scientists create a functioning gene to replace the faulty one in the retina. Then they place the new gene inside a little “coat” made up of viral proteins (known as a vector). The type of virus they use does not have the ability to reproduce or cause disease. To administer LUXTURNA, doctors add the new gene by injecting it directly into the eye through a thin needle (see 1 in illustration) connected to a syringe, with the help of a light probe (2). The new gene (3) enters the cell nucleus (4), where it makes the healthy enzymes (5) required to see.
A breakthrough restores Hannah’s sight
Hannah had the gene therapy in her left eye on July 10 – and her world changed overnight. “Starting 24 hours after her surgery, her treated eye was very sensitive to light,” says Amy. “She came downstairs and flipped on her little desk lamp on our kitchen table like she always does, and then she pushed it away — it shocked her! No light had ever been too bright before.”
Those breakthrough moments kept happening. Hannah could see the buttons on the microwave. She could see the liquid in her drinking glass. She could see the buttons on the console in the backseat of the family’s car. She could see rain.
“The whole family is really appreciating these moments,” says Chris. “She’s seeing things for the first time.”
Hannah had her second eye treated on July 23. And when her family arrived in Ocean City, NJ, for vacation a few weeks later, her parents noticed a huge difference right away.
“In the past, whenever we went to a new shore house, she would struggle to find her way around initially,” says Amy. “She would walk in the door and put her hands out. But this year there was none of that. She walked in and was able to navigate her way around very easily.”
Looking ahead, thanks to LUXTURNA
Hannah was the first patient to receive LUXTURNA at CHOP after it was approved by the FDA. And because LUXTURNA is a new treatment, there are still many unknowns about what the future holds for her. Her care team, including Dr. Maguire and pediatric ophthalmologist Bart Leroy, MD, PhD, will follow her closely. And she’ll have her family by her side as the world continues to reveal itself to her — moment by moment, day by day.
“Think about it,” says Chris. “When was the first time you saw a star? None of us remember that. But for Hannah, it’s a moment she’ll never forget.”