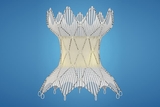
CHOP was lead enrolling site in clinical trial that led to approval of Harmony™ Transcatheter Pulmonary Valve (TPV), which allows for pulmonary valve replacement via a minimally invasive catheter-based procedure.
The Food and Drug Administration (FDA) has approved the Harmony™ Transcatheter Pulmonary Valve (TPV) to treat patients with congenital heart disease who have chronic pulmonary valve regurgitation. The device consists of a self-expanding metal frame combined with valve leaflets that can be implanted inside a patient’s heart using a catheter-based delivery system during a cardiac catheterization.
Patients with congenital heart disease (CHD), which affects an estimated 40,000 infants each year and is the most common birth defect in the United States, often face numerous open-heart surgeries over their lifetime to correct issues related to their dysfunctional pulmonary valve. However, open-heart surgery poses risks to the patient, and the procedure requires months of recovery.
Using a transcatheter allows for a less invasive approach, whereby a transcatheter procedure is used to replace the pulmonary valve. The procedure requires less anesthesia, reduces the risk of infection, and leads to a faster recovery. Transcatheter therapies were typically only performed in adults, but this new device has adapted the procedure for use in children.
"The approval of this device will allow us to provide a non-surgical option to patients with CHD, reducing or eliminating the need for multiple open-heart surgeries over the course of a patient’s lifetime," said Matthew J. Gillespie, MD, interventional cardiologist in the Cardiac Center, director of the Cardiac Catheterization Laboratory at CHOP and co-director of the Pediatric Heart Valve Center. “Researchers in the Cardiac Center at CHOP are committed to bringing our expertise to the development of cutting-edge treatments, which will improve the lives of our patients and their families."
Read more about our patients who received the Harmony Transcatheter Pulmonary Valve in preclinical trials: Mac and Casey
Featured in this article
Specialties & Programs
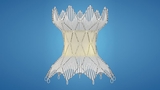
CHOP was lead enrolling site in clinical trial that led to approval of Harmony™ Transcatheter Pulmonary Valve (TPV), which allows for pulmonary valve replacement via a minimally invasive catheter-based procedure.

The Food and Drug Administration (FDA) has approved the Harmony™ Transcatheter Pulmonary Valve (TPV) to treat patients with congenital heart disease who have chronic pulmonary valve regurgitation. The device consists of a self-expanding metal frame combined with valve leaflets that can be implanted inside a patient’s heart using a catheter-based delivery system during a cardiac catheterization.
Patients with congenital heart disease (CHD), which affects an estimated 40,000 infants each year and is the most common birth defect in the United States, often face numerous open-heart surgeries over their lifetime to correct issues related to their dysfunctional pulmonary valve. However, open-heart surgery poses risks to the patient, and the procedure requires months of recovery.
Using a transcatheter allows for a less invasive approach, whereby a transcatheter procedure is used to replace the pulmonary valve. The procedure requires less anesthesia, reduces the risk of infection, and leads to a faster recovery. Transcatheter therapies were typically only performed in adults, but this new device has adapted the procedure for use in children.
"The approval of this device will allow us to provide a non-surgical option to patients with CHD, reducing or eliminating the need for multiple open-heart surgeries over the course of a patient’s lifetime," said Matthew J. Gillespie, MD, interventional cardiologist in the Cardiac Center, director of the Cardiac Catheterization Laboratory at CHOP and co-director of the Pediatric Heart Valve Center. “Researchers in the Cardiac Center at CHOP are committed to bringing our expertise to the development of cutting-edge treatments, which will improve the lives of our patients and their families."
Read more about our patients who received the Harmony Transcatheter Pulmonary Valve in preclinical trials: Mac and Casey
Contact us
Natalie Solimeo
Topolewski Pediatric Heart Valve Center