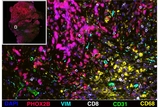
Researchers at Children’s Hospital of Philadelphia (CHOP) developed a longitudinal atlas of neuroblastoma, a common and potentially deadly childhood cancer, to gain deeper understanding into precise molecular mechanisms underlying why and how certain treatments eventually become ineffective. The findings, which offer insights that could potentially lead to new personalized medicine approaches in neuroblastoma treatment, were published today in the journal Nature Genetics.
Despite significant advances in the standard of care, the 5-year survival rate of high-risk neuroblastoma after diagnosis remains less than 50%. Neuroblastoma cells within the same tumor can vary greatly, which creates challenges in treatment efficacy. Until now, the scientific community lacked understanding of how the tumor microenvironment changes during treatment. In this study, researchers created a cell atlas that afforded the first in-depth look at how different cell types, like malignant cells and immune cells, interact and change within their natural environment, which led to valuable new insights.

“Our atlas provides a crucial foundation for developing novel treatments by mapping the complex interactions between malignant cells and surrounding cells that support tumor growth,” said senior study author Kai Tan, PhD, a professor in the Department of Pediatrics at Children’s Hospital of Philadelphia who spearheads CHOP’s participation in the National Cancer Institute (NCI) Human Tumor Atlas Network (HTAN). “As researchers, we look to use these insights to tailor therapies to target unique characteristics of a patient’s tumor. Overall, we are optimistic about the doors our research and techniques are opening.”
In this study, CHOP researchers used advanced single cell sequencing and spatial omics techniques to analyze the transcriptional, epigenetic and proteomic profiles of tumor samples from 22 pediatric patients with high-risk neuroblastoma before and after induction chemotherapy. This multidimensional dataset can be accessed from the HTAN data portal.
The authors uncovered diverse tumor and non-tumor cell characteristics, as well as major changes in these characteristics following chemotherapy. They found that patients had worse outcomes when cancer cells were multiplying more actively and became more metabolically active. Conversely, tumors that developed more mature, neuron-like features resulted in better outcomes in patients. Additionally, researchers found that an increase in a type of mesenchymal-like tumor cells was linked to poorer responses in chemotherapy. Certain immune cells (e.g. macrophages) became more active in ways that led to tumor growth by enabling blood vessel growth while suppressing the immune response.
Researchers also discovered a specific communication pathway (HB-EGF/ERBB4) between macrophages and cancer cells that triggered signals promoting tumor growth. These findings highlight the importance of identifying new factors inside the tumor’s microenvironment that influence how high-risk neuroblastoma responds to treatment. Using preclinical models, the researchers are further testing this pathway with the goal of eventually translating the findings to novel therapeutic strategies.

“Studies of this magnitude are only made possible by monumental team efforts,” said Wenbao Yu, PhD, a lead author of the study and a Research Assistant Professor at Children’s Hospital of Philadelphia. “With the collaboration of biologists, clinicians, and computational scientists, we were able to gain new insights into the complex ecosystem of neuroblastoma.”
The research was supported by the National Cancer Institute (NCI) Human Tumor Atlas Network grant under award (#U2C CA233285). Additional support includes National Institutes of Health (NIH) grant (U54 HL165442), American Cancer Society Institutional Research Grant (IRG-22-150- 41-IRG) and NIH grant (T32 CA009140).
Yu et al. “Longitudinal single-cell multiomic atlas of high-risk neuroblastoma reveals chemotherapy-induced tumor microenvironment rewiring. Nature Genetics. Online April 14, 2025. DOI: 10.1038/s41588-025-02158-6.
Featured in this article
Experts
Specialties & Programs
Research
Researchers at Children’s Hospital of Philadelphia (CHOP) developed a longitudinal atlas of neuroblastoma, a common and potentially deadly childhood cancer, to gain deeper understanding into precise molecular mechanisms underlying why and how certain treatments eventually become ineffective. The findings, which offer insights that could potentially lead to new personalized medicine approaches in neuroblastoma treatment, were published today in the journal Nature Genetics.
Despite significant advances in the standard of care, the 5-year survival rate of high-risk neuroblastoma after diagnosis remains less than 50%. Neuroblastoma cells within the same tumor can vary greatly, which creates challenges in treatment efficacy. Until now, the scientific community lacked understanding of how the tumor microenvironment changes during treatment. In this study, researchers created a cell atlas that afforded the first in-depth look at how different cell types, like malignant cells and immune cells, interact and change within their natural environment, which led to valuable new insights.

“Our atlas provides a crucial foundation for developing novel treatments by mapping the complex interactions between malignant cells and surrounding cells that support tumor growth,” said senior study author Kai Tan, PhD, a professor in the Department of Pediatrics at Children’s Hospital of Philadelphia who spearheads CHOP’s participation in the National Cancer Institute (NCI) Human Tumor Atlas Network (HTAN). “As researchers, we look to use these insights to tailor therapies to target unique characteristics of a patient’s tumor. Overall, we are optimistic about the doors our research and techniques are opening.”
In this study, CHOP researchers used advanced single cell sequencing and spatial omics techniques to analyze the transcriptional, epigenetic and proteomic profiles of tumor samples from 22 pediatric patients with high-risk neuroblastoma before and after induction chemotherapy. This multidimensional dataset can be accessed from the HTAN data portal.
The authors uncovered diverse tumor and non-tumor cell characteristics, as well as major changes in these characteristics following chemotherapy. They found that patients had worse outcomes when cancer cells were multiplying more actively and became more metabolically active. Conversely, tumors that developed more mature, neuron-like features resulted in better outcomes in patients. Additionally, researchers found that an increase in a type of mesenchymal-like tumor cells was linked to poorer responses in chemotherapy. Certain immune cells (e.g. macrophages) became more active in ways that led to tumor growth by enabling blood vessel growth while suppressing the immune response.
Researchers also discovered a specific communication pathway (HB-EGF/ERBB4) between macrophages and cancer cells that triggered signals promoting tumor growth. These findings highlight the importance of identifying new factors inside the tumor’s microenvironment that influence how high-risk neuroblastoma responds to treatment. Using preclinical models, the researchers are further testing this pathway with the goal of eventually translating the findings to novel therapeutic strategies.

“Studies of this magnitude are only made possible by monumental team efforts,” said Wenbao Yu, PhD, a lead author of the study and a Research Assistant Professor at Children’s Hospital of Philadelphia. “With the collaboration of biologists, clinicians, and computational scientists, we were able to gain new insights into the complex ecosystem of neuroblastoma.”
The research was supported by the National Cancer Institute (NCI) Human Tumor Atlas Network grant under award (#U2C CA233285). Additional support includes National Institutes of Health (NIH) grant (U54 HL165442), American Cancer Society Institutional Research Grant (IRG-22-150- 41-IRG) and NIH grant (T32 CA009140).
Yu et al. “Longitudinal single-cell multiomic atlas of high-risk neuroblastoma reveals chemotherapy-induced tumor microenvironment rewiring. Nature Genetics. Online April 14, 2025. DOI: 10.1038/s41588-025-02158-6.
Contact us
Jennifer Lee
Cancer Center