Brain development is one of the most complicated processes that occurs during in utero development. During fetal life, the developing brain is normally exposed to lower oxygen levels than are present in ambient air. However, when there is an even lower amount of oxygen than expected, this can lead to significant brain injury. Hypoxia can affect 1 to 6 in 1,000 pregnancies each year. Problems from hypoxia can include developmental delays, learning difficulties, autism and epilepsy. Brain magnetic resonance imaging (MRI) shows that there is injury to the brain’s gray and white matter.
The causes of hypoxia are variable and can occur chronically from conditions that cause placental insufficiency, congenital heart disease, or acutely from placental abruption, cord compression or uterine rupture
After birth, we usually provide these children with comprehensive supportive care to treat any neurological complications. This would include developmental support, early intervention therapies, or treatment of seizures. However, we still do not have specific therapies that can help improve or prevent neurodevelopmental disease.
Developing novel models
There are some limitations to current models. So, to better understand how low oxygen levels affect brain development, we have crafted a novel preclinical model of acute hypoxic injury in mice. In this model, we have shown deficits similar to long-term neuroanatomical and behavioral deficits seen in children with hypoxic injury. While we have been studying mice, we have used similar studies used in humans to be able to translate findings. This includes working with Neuropathology and Neuroradiology. Brain MRI and histology shows these mice have white and gray matter injury similar to what is seen in humans. We are also working to better understand the more subtle differences in behavior and learning in children with hypoxic brain injury by comparing multiple model conditions.
Goals for the future
By trying to model human hypoxic conditions in mice more precisely using a combination of state-of-the-art molecular biology, metabolomics and proteomics techniques, we aim to:
- Develop novel treatment strategies to improve developmental outcomes
- Determine if there are biomarkers to help predict which children with hypoxic injury during pregnancy or birth may need extra therapy
- Test if there are genetic or environmental factors that predispose children with increased injury or specific deficits from hypoxia
We will then study all our findings in patients with different kinds of hypoxic injury and other forms of in utero brain injury.
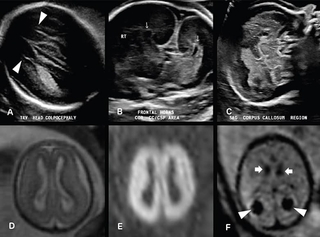
Above images: 22-week fetus with absent cavum septi pellucidi. Transverse US image of the lateral ventricles (A) demonstrates a parallel configuration of the ventricles with a pointed configuration of the anterior horns (arrowheads), consistent with colpocephaly. Coronal US image (B) through the lateral ventricle frontal horns demonstrates absence of the cavum septi pellucidi and an abnormal “trident” configuration of the frontal horns (arrows). Midline sagittal US image (C) reveals no identifiable corpus callosum. The constellation of findings are characteristic of complete agenesis of the corpus callosum.
Fetal brain MRI of 19-week surviving twin of monochorionic diamniotic pregnancy. Axial T2-weighted image (D) demonstrates diffuse abnormally increased signal throughout both cerebral hemispheres. Axial diffusion weighted image (E) reveals extensive restricted diffusion throughout both cerebral hemispheres, consistent with extensive hypoxic ischemic injury. Axial echoplanar image (F) reveals susceptibility from hemosiderin deposition in both choroid plexuses (arrowheads) and germinal matrices (arrows), consistent with bilateral choroid plexus and germinal matrix hemorrhages.
Featured in this article
Specialties & Programs
Brain development is one of the most complicated processes that occurs during in utero development. During fetal life, the developing brain is normally exposed to lower oxygen levels than are present in ambient air. However, when there is an even lower amount of oxygen than expected, this can lead to significant brain injury. Hypoxia can affect 1 to 6 in 1,000 pregnancies each year. Problems from hypoxia can include developmental delays, learning difficulties, autism and epilepsy. Brain magnetic resonance imaging (MRI) shows that there is injury to the brain’s gray and white matter.
The causes of hypoxia are variable and can occur chronically from conditions that cause placental insufficiency, congenital heart disease, or acutely from placental abruption, cord compression or uterine rupture
After birth, we usually provide these children with comprehensive supportive care to treat any neurological complications. This would include developmental support, early intervention therapies, or treatment of seizures. However, we still do not have specific therapies that can help improve or prevent neurodevelopmental disease.
Developing novel models
There are some limitations to current models. So, to better understand how low oxygen levels affect brain development, we have crafted a novel preclinical model of acute hypoxic injury in mice. In this model, we have shown deficits similar to long-term neuroanatomical and behavioral deficits seen in children with hypoxic injury. While we have been studying mice, we have used similar studies used in humans to be able to translate findings. This includes working with Neuropathology and Neuroradiology. Brain MRI and histology shows these mice have white and gray matter injury similar to what is seen in humans. We are also working to better understand the more subtle differences in behavior and learning in children with hypoxic brain injury by comparing multiple model conditions.
Goals for the future
By trying to model human hypoxic conditions in mice more precisely using a combination of state-of-the-art molecular biology, metabolomics and proteomics techniques, we aim to:
- Develop novel treatment strategies to improve developmental outcomes
- Determine if there are biomarkers to help predict which children with hypoxic injury during pregnancy or birth may need extra therapy
- Test if there are genetic or environmental factors that predispose children with increased injury or specific deficits from hypoxia
We will then study all our findings in patients with different kinds of hypoxic injury and other forms of in utero brain injury.

Above images: 22-week fetus with absent cavum septi pellucidi. Transverse US image of the lateral ventricles (A) demonstrates a parallel configuration of the ventricles with a pointed configuration of the anterior horns (arrowheads), consistent with colpocephaly. Coronal US image (B) through the lateral ventricle frontal horns demonstrates absence of the cavum septi pellucidi and an abnormal “trident” configuration of the frontal horns (arrows). Midline sagittal US image (C) reveals no identifiable corpus callosum. The constellation of findings are characteristic of complete agenesis of the corpus callosum.
Fetal brain MRI of 19-week surviving twin of monochorionic diamniotic pregnancy. Axial T2-weighted image (D) demonstrates diffuse abnormally increased signal throughout both cerebral hemispheres. Axial diffusion weighted image (E) reveals extensive restricted diffusion throughout both cerebral hemispheres, consistent with extensive hypoxic ischemic injury. Axial echoplanar image (F) reveals susceptibility from hemosiderin deposition in both choroid plexuses (arrowheads) and germinal matrices (arrows), consistent with bilateral choroid plexus and germinal matrix hemorrhages.
Contact us
Richard D. Wood Jr. Center for Fetal Diagnosis and Treatment