
Two years ago, the Division of Urology at Children’s Hospital of Philadelphia (CHOP) established one of the country’s first percutaneous posterior tibial nerve stimulation (PTNS) pathways for pediatric lower urinary tract dysfunction (LUTD). Under the direction of Jason Van Batavia, MD, MSTR and Adriana Messina, MSN, CRNP, FNP-BC, the PTNS program has already impacted the lives of over 30 CHOP pediatric patients.
LUTD studies and treatment
LUTD, also known as bladder dysfunction, is one of the most common reasons for referral to the pediatric urologist. Multiple studies across races and ethnicities show a similar LUTD prevalence rate of 20-30% in school age children and adolescents. The incidence of LUTD likely increases with age and adult series show rates as high as 40% among 40-year-olds. Furthermore, the literature on LUTD supports the notion that untreated LUTD in childhood can persist and worsen in adulthood, often leading to debilitating conditions like chronic bladder pain syndrome or interstitial cystitis. Therefore, treating LUTD in children and adolescents is a critical part of pediatric urology practice and can have lifelong implications.
The standard treatment for pediatric LUTD follows a three-tiered approach:
- The first tier is urotherapy (i.e., education, behavioral modifications, timed voiding and intake/elimination diary tracking).
- The second tier includes biofeedback, pelvic floor physical therapy and medications - most commonly antimuscarinics or beta-3 agonists which treat overactive bladder.
- For children and adolescents with refractory LUTD, there is a third tier of therapeutic options including neuromodulation. Neuromodulation ranges from the minimally invasive sacral transcutaneous electrical stimulation (TENS) to the most invasive implantation of a sacral nerve stimulator (SNS). While TENS is likely less efficacious compared to SNS, the use of SNS is not FDA-approved for patients under the age of 16 years old and studies have shown a high complication and re-operation rate (>60%) when used off-label in children and young adolescents. An intermediate option between TENS and SNS is PTNS.
How PTNS treatment works
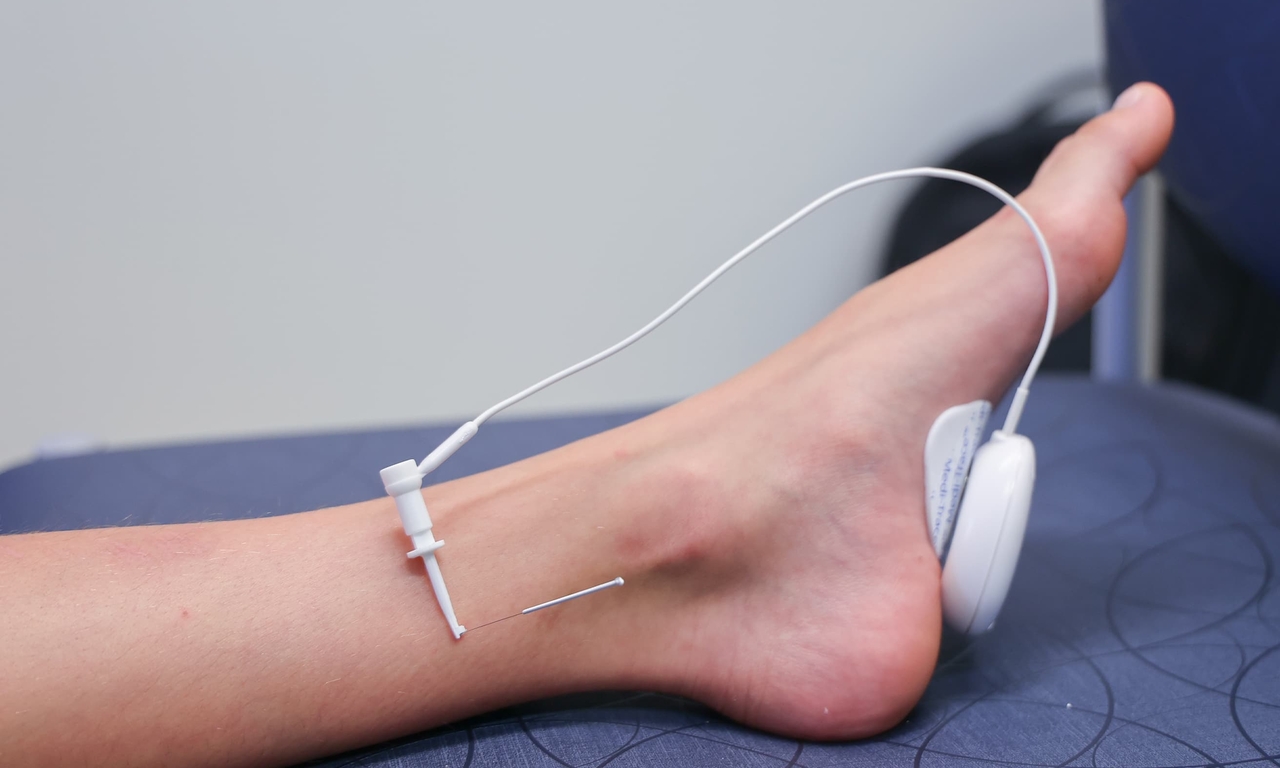
PTNS involves placement of a 34-gauge needle in the lower leg just cephalad and posterior to the medial malleolus. The needle is connected to an external stimulator placed on the sole of the foot with a sticker. The needle delivers electrical stimulation close to the posterior tibial nerve, which is a mixed motor and sensory nerve from the spinal L4-S3 roots. These same nerve roots are also the spinal levels from which the nerves that innervate the bladder arise and while the exact mechanism of action is unknown, the stimulation is thought to help regulate excitatory and inhibitory signaling affecting bladder function.
PTNS can only be performed in the office under the supervision of a trained physician or advanced practice provider and the induction protocol involves weekly 30-minute sessions for 12 consecutive weeks, followed by maintenance sessions every other week or monthly for up to two years.
Improved quality of life
Despite PTNS being approved by the FDA for “urinary urgency/frequency and urge incontinence” since 2000, there are few studies in the United States on the use of PTNS for pediatric LUTD and the majority of publications are in adult populations. Ideal candidates for PTNS are children and adolescents with refractory overactive bladder symptoms (i.e., urgency, frequency and urge incontinence) who have failed previous medication therapy and/or biofeedback. Patients do not need spinal MRIs or invasive urodynamic procedures prior to starting PTNS and our preliminary data show that 80% of patients have improved symptom score and quality of life measures. We have been amazed at how well our pediatric patients tolerate needle placement and for those who are anxious, we have used topical analgesics and vibration devices (“Buzzy Bee”) for distraction.
Looking to the future for LUTD patients
We are reviewing our data for improvements in other symptoms besides urgency and daytime incontinence and have noted significant improvements in nocturnal enuresis and bowel dysfunction. We are encouraged by these promising results and by the overwhelmingly grateful and enthusiastic support of our patients who have undergone PTNS and their families. In fact, despite the time intensity of the induction period for PTNS, we have had families drive more than two hours round trip week in and week out to continue therapy. We are proud to be one of the few urology divisions across the country that is offering this neuromodulation therapy to pediatric patients and will continue to look for ways to bring state-of-the-art techniques to our LUTD patients.
Two years ago, the Division of Urology at Children’s Hospital of Philadelphia (CHOP) established one of the country’s first percutaneous posterior tibial nerve stimulation (PTNS) pathways for pediatric lower urinary tract dysfunction (LUTD). Under the direction of Jason Van Batavia, MD, MSTR and Adriana Messina, MSN, CRNP, FNP-BC, the PTNS program has already impacted the lives of over 30 CHOP pediatric patients.
LUTD studies and treatment
LUTD, also known as bladder dysfunction, is one of the most common reasons for referral to the pediatric urologist. Multiple studies across races and ethnicities show a similar LUTD prevalence rate of 20-30% in school age children and adolescents. The incidence of LUTD likely increases with age and adult series show rates as high as 40% among 40-year-olds. Furthermore, the literature on LUTD supports the notion that untreated LUTD in childhood can persist and worsen in adulthood, often leading to debilitating conditions like chronic bladder pain syndrome or interstitial cystitis. Therefore, treating LUTD in children and adolescents is a critical part of pediatric urology practice and can have lifelong implications.
The standard treatment for pediatric LUTD follows a three-tiered approach:
- The first tier is urotherapy (i.e., education, behavioral modifications, timed voiding and intake/elimination diary tracking).
- The second tier includes biofeedback, pelvic floor physical therapy and medications - most commonly antimuscarinics or beta-3 agonists which treat overactive bladder.
- For children and adolescents with refractory LUTD, there is a third tier of therapeutic options including neuromodulation. Neuromodulation ranges from the minimally invasive sacral transcutaneous electrical stimulation (TENS) to the most invasive implantation of a sacral nerve stimulator (SNS). While TENS is likely less efficacious compared to SNS, the use of SNS is not FDA-approved for patients under the age of 16 years old and studies have shown a high complication and re-operation rate (>60%) when used off-label in children and young adolescents. An intermediate option between TENS and SNS is PTNS.
How PTNS treatment works

PTNS involves placement of a 34-gauge needle in the lower leg just cephalad and posterior to the medial malleolus. The needle is connected to an external stimulator placed on the sole of the foot with a sticker. The needle delivers electrical stimulation close to the posterior tibial nerve, which is a mixed motor and sensory nerve from the spinal L4-S3 roots. These same nerve roots are also the spinal levels from which the nerves that innervate the bladder arise and while the exact mechanism of action is unknown, the stimulation is thought to help regulate excitatory and inhibitory signaling affecting bladder function.
PTNS can only be performed in the office under the supervision of a trained physician or advanced practice provider and the induction protocol involves weekly 30-minute sessions for 12 consecutive weeks, followed by maintenance sessions every other week or monthly for up to two years.
Improved quality of life
Despite PTNS being approved by the FDA for “urinary urgency/frequency and urge incontinence” since 2000, there are few studies in the United States on the use of PTNS for pediatric LUTD and the majority of publications are in adult populations. Ideal candidates for PTNS are children and adolescents with refractory overactive bladder symptoms (i.e., urgency, frequency and urge incontinence) who have failed previous medication therapy and/or biofeedback. Patients do not need spinal MRIs or invasive urodynamic procedures prior to starting PTNS and our preliminary data show that 80% of patients have improved symptom score and quality of life measures. We have been amazed at how well our pediatric patients tolerate needle placement and for those who are anxious, we have used topical analgesics and vibration devices (“Buzzy Bee”) for distraction.
Looking to the future for LUTD patients
We are reviewing our data for improvements in other symptoms besides urgency and daytime incontinence and have noted significant improvements in nocturnal enuresis and bowel dysfunction. We are encouraged by these promising results and by the overwhelmingly grateful and enthusiastic support of our patients who have undergone PTNS and their families. In fact, despite the time intensity of the induction period for PTNS, we have had families drive more than two hours round trip week in and week out to continue therapy. We are proud to be one of the few urology divisions across the country that is offering this neuromodulation therapy to pediatric patients and will continue to look for ways to bring state-of-the-art techniques to our LUTD patients.