Types of Vaccine Ingredients
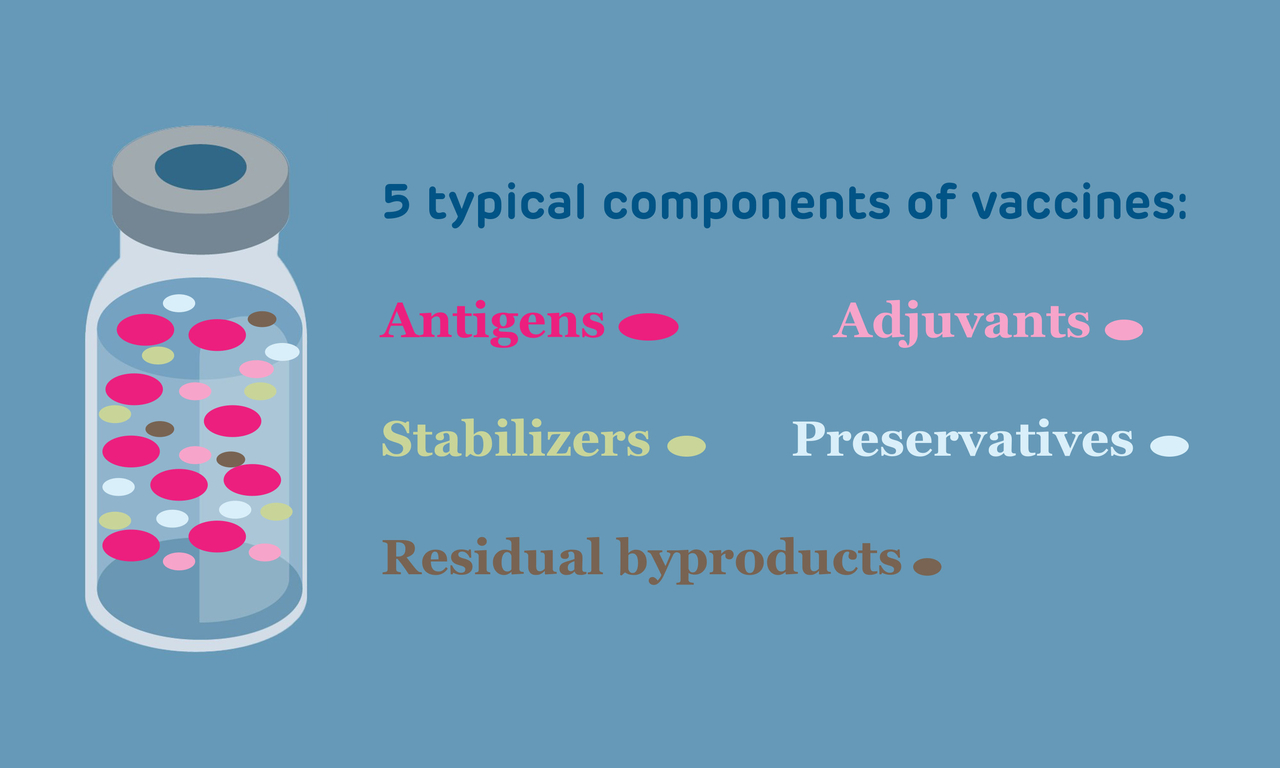
Antigens
Antigens can be considered the active ingredients in vaccines because they are the parts of the vaccine to which an immune response is generated. Antigens are one of the following types:
- Whole viruses or bacteria
- Parts of the viruses or bacteria
- Products made by bacteria, called toxins
- Nucleic acids
Learn more about how vaccines are made.
Adjuvants
Adjuvants are substances that allow vaccines to work better by enhancing the immune response to the vaccine, decreasing the quantity of vaccine needed to gain protective immunity, or lowering the number of doses required.
Currently, five types of adjuvants are approved for use in the United States:
- Aluminum salts — Salts that contain aluminum have been used in vaccines since the 1930s. Find out which vaccines contain aluminum, how much is present, and how we know aluminum in vaccines is safe.
- Monophosphoryl lipid A — This substance was isolated from the surface of bacteria. It is modified so that it is not harmful when added to vaccines. The vaccines in the U.S. that currently use this adjuvant are the shingles vaccine (called Shingrix), one of the RSV vaccines (called Arexvy), and the protein-based COVID-19 vaccine (Nuvaxovid).
- QS21— Isolated from the bark of Quillaja saponaria trees, this molecule is soap-based. It is found in the shingles vaccine (called Shingrix), one of the RSV vaccines (called Arexvy), and the protein-based COVID-19 vaccine (Nuvaxovid).
- MF59 — A substance composed of squalene oil emulsified in water. Squalene is found in people, animals and plants. It is used in the U.S. in a version of the influenza vaccine (called FLUAD). Because squalene is an oil-in-water emulsion, some people wonder whether they can get vaccines if they have allergies to peanut or corn oils. However, these oils are not in vaccines.
- CpG — Two nucleotides, Cytosine (C) and Guanine (G), which are building blocks of DNA, are linked to form an adjuvant contained in one of the hepatitis B vaccines (called Heplisav-B).
Stabilizers
Stabilizers are used in vaccines to protect the integrity of the active ingredients during manufacture, storage and transport. A commonly used stabilizer that can be associated with allergic responses to vaccines is gelatin.
Another common stabilizer is polysorbate 80. It can also be present in very low quantities as a residual byproduct.
* A microgram is one-millionth of a gram, and a gram is the weight of one-fifth of a teaspoon of water.
Preservatives
Preservatives are used in some vaccines to prevent bacterial or fungal contamination. The requirement for preservatives in vaccines arose from many incidents that occurred in children in the early 20th century. After getting vaccines contained in multi-dose vials, these children developed severe and occasionally fatal bacterial infections. Healthcare providers realized that sometimes when putting a needle in the top of the vial to draw up a dose of vaccine, bacteria would accidentally be introduced. The child who got the current dose was fine, but the child who got the next dose would also be inoculated with the contaminating bacteria. If the bacteria could cause harm and if it was present in a large enough quantity, the child would have health effects caused by the contaminant. For example, in 1916, four children died, 26 developed local abscesses, and 68 developed severe systemic infections after receipt of a typhoid vaccine contaminated with the bacteria Staphylococcus aureus. Preservatives prevent these inadvertent contaminants. As a consequence of these incidents, preservatives have been required for vaccines contained in multi-dose vials (with some exceptions) since the 1930s.
Thimerosal, a mercury-containing preservative used in vaccines, has been the focus of intense scrutiny. Thimerosal is no longer used as a preservative in any routinely used vaccines in the U.S., but it is used in vaccines distributed in multi-dose vials in some other countries. Because of fears that thimerosal contained in vaccines could harm children, numerous studies have been conducted to evaluate this concern. These large, controlled studies have not found evidence of harm. To read about some of these studies, go to the "Thimerosal (mercury) and vaccines" section of our vaccine safety references page.
Manufacturing byproducts
Because vaccines are made from viruses and bacteria, some chemicals and cell byproducts used during vaccine production may remain in the final preparation in small quantities. Some examples include antibiotics, DNA, egg proteins, fetal tissues, formaldehyde, human proteins, and yeast.
Concerns have also been voiced about SV40, a virus found in cells used to grow the poliovirus vaccine during the 1950s and 1960s. However, these concerns were proven to be unfounded, and SV40 is not present in any of today’s vaccines.
References
Adjuvants other than aluminum in vaccines
For a list of studies related to aluminum in vaccines, visit the aluminum page.
Squalene (MF59, AS03, AF03)
Weibel D, Sturkenboom M, Black, S, et al. Narcolepsy and adjuvanted pandemic influenza A (H1N1) 2009 vaccines — multi-country assessment. Vaccine 2018; 38:6202.
The authors found no increased risk of narcolepsy in children or adults in Argentina, Canada, Spain, Switzerland, Taiwan and the Netherlands following implementation of squalene-adjuvanted (AS03, MF59) influenza H1N1 vaccines in those countries.
Dos Santos G, Seifert HA, Bauchau V, Shinde V, Barbeau DM, et al. Adjuvanted (AS03) A/H1N1 2009 Pandemic influenza vaccines and solid organ transplant rejection: systematic signal evaluation and lessons learnt. Drug Saf 2017;40:693-702.
In 2010, the European Medicines Agency (EMA) requested an assessment of available data following a signal of solid organ transplant (SOT) rejection after immunization with either of GlaxoSmithKline’s (GSK) two adjuvanted (AS03) pandemic influenza vaccines, Pandemrix® and Arepanrix® H1N1. The authors investigated 5,000 patients who received the 2009 A/H1N1 pandemic vaccine and an additional 11,000 subjects who received adjuvanted (AS03) A/H5N1 vaccines. They found that data supported the safety of adjuvanted (AS03) pandemic influenza vaccination in SOT recipients.
Kumar D, Campbell P, Hoschler K, Hildago L, Al-Dabbagh M, et al. Randomized controlled trial of adjuvanted versus non adjuvanted influenza vaccine in kidney transplant patients. Transplantation 2016;100;662-669.
The authors compared the safety and immunogenicity of the 2012-2013 influenza vaccine with or without MF59 adjuvant in adult patients who had received a kidney transplant within a median time of 8.1 years. They found no significant differences in response to the vaccine between groups and no evidence of HLA upregulation in transplant recipients who received adjuvanted vaccine.
Stassijns J, Bollaerts K, Baay M, Verstraeten T. A systematic review and meta-analysis on the safety of newly adjuvanted vaccines among children. Vaccine 2016; 34:714-722.
The authors conducted a systematic review on the safety of newly adjuvanted vaccines in more than 25,000 children ≤ 10 years of age, specifically those containing AS01, AS02, AS03 and MF59. They found that serious adverse events did not occur more frequently in the groups receiving adjuvanted vaccines.
Vesikari T, Forsten A, Arora A, Tsai T, Clemens R. Influenza vaccination in children primed with MF59-adjuvanted or non-adjuvanted seasonal influenza vaccine. Hum Vaccin Immunother 2015;11(8):2102-2112.
The authors compared the safety and immunogenicity of vaccination with trivalent inactivated influenza vaccine (TIV) versus MF59-adjuvanted TIV (aTIV) in children. They found that aTIV produced a significantly more robust immune response and a slightly higher but acceptable local and systemic reactions compared with TIV.
Rubinstein F, Micone P, Bonotti A, Wainer V, Schwarcz A, et al. Influenza A/H1N1 MF59 adjuvanted vaccine in pregnant women and adverse perinatal outcomes: multicenter study. BMJ 2013;346:f393.
Approximately 30,000 pregnant women, including more than 7,000 vaccinated during pregnancy, and their newborns were evaluated to assess the risk of adverse perinatal events (fetal and maternal) associated with receipt of MF59-adjuvanted influenza vaccine. Vaccinated women had a significantly lower risk of preterm birth (< 37 weeks), low birth weight, early neonatal mortality, perinatal mortality (early neonatal mortality plus fetal mortality), low APGAR scores, infant non-immune jaundice, hospital admission during pregnancy, and first trimester hemorrhage compared with women who were not vaccinated during pregnancy.
Siegrist CA, Ambrosioni J, Bel M, Combescure C, Hadaya K, et al. Responses of solid organ transplant recipients to the AS03-adjuvanted pandemic influenza vaccine. Antivir Ther 2012;17(5):893-903.
During the 2009 H1N1 pandemic, solid organ transplant (SOT) recipients in Switzerland received two doses of an AS03-adjuvanted influenza vaccine (Pandemrix). The authors compared the safety and immunogenicity of two doses of AS03-adjuvanted vaccine in more than 200 SOT recipients (heart, lung, liver, kidney, pancreas/islet of Langerhans) to one dose in healthy family controls. Adverse reactions occurred less frequently in the SOT group and SOT graft function remained unaffected.
Black S, Della Cioppa G, Malfroot A, Nacci P, Nicolay U, et al. Safety of MF59-adjuvanted versus non-adjuvanted influenza vaccines in children and adolescents: an integrated analysis. Vaccine 2010;28:7331-7336.
The authors investigated the safety profile of MF59-adjuvanted and non-adjuvanted seasonal and pandemic influenza vaccines in more than 1,700 subjects aged 6 months to 18 years. Subjects in the MF59-adjuvanted group experienced a higher percentage of local and systemic reactions, such as injection site reactions or irritability. The group receiving adjuvanted influenza vaccines did not experience an increase in the incidence of serious adverse events.
Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009;27:6959-6965.
The authors evaluated safety data from 64 clinical trials involving 20,000 recipients of MF59-containing seasonal and pandemic influenza vaccines. Other than an increased risk of local and systemic reactions such as injection site reactions or irritability, vaccine recipients had a lower risk of cardiovascular events, new onset chronic diseases, or death.
Monophosphoryl lipid A (MPL), saponin (QS21), and related adjuvant systems (AS01, AS02, AS04)
Bigaeva E, van Doorn E, Liu H, Hak E. Meta-analysis on randomized controlled trials of vaccines with QS-21 or ISCOMATRIX adjuvant: safety and tolerability. PLoSONE 2016;11(5):e0154757.
Saponin is an immunostimulant that is extracted from the bark of a South American tree, Quillaja sapnonaria. Two saponin-based adjuvants are QS21 and ISCOMATRIX. The authors conducted a systematic literature review to assess the safety and tolerability of saponin-based adjuvants and found no increase in the incidence of reported systemic adverse events.
Leroux-Roels G, Leroux-Roels I, Clement F, Ofori-Anyinam O, Lievens M, et al. Evaluation of the immune response to RTS,S/AS01 and RT,S/AS02 adjuvanted vaccines. Hum Vacc Immunother 2014;10(8):2211-2219.
The authors compared the safety and immunogenicity of malaria vaccines adjuvanted with squalene, monophosphoryl lipid A and saponin to a non-adjuvanted malaria vaccine in adults and found greater immune responses with adjuvanted vaccines. Adjuvanted vaccines had a higher rate of injection site reactions and certain generalized reactions (e.g., fatigue, headache) compared with the non-adjuvanted vaccine but within acceptable limits. These side effects typically resolved within seven days. No serious adverse events were reported for any group.
Toft L, Storgaard M, Muller M, Sehr P, Bonde P, et al. Comparison of the immunogenicity and reactogenicity of Cervarix and Gardasil human papillomavirus vaccines in HIV-infected adults: a randomized, double-blind clinical trial. J Infect Dis 2014;209:1165-1173.
The immune response and safety of Cervarix®, a monophosphoryl lipid A-containing-HPV vaccine with Gardasi®l, an aluminum-adjuvanted HPV vaccine were compared in HIV infected adults. The authors found that Cervarix induced superior vaccine responses in HIV-infected women. Both vaccines were well tolerated, though Cervarix was associated with a higher rate of injection site reactions. No serious adverse events occurred in either group.
Wald A, Koelle DM, Fife K, Warren T, Leclair K, et al. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine 2011;29(47):8520-8529.
A candidate vaccine, HerpV, against herpes simplex virus-2 (HSV-2) was tested for safety and immunogenicity in a phase 1 human adult study. The authors compared HerpV + QS21 (saponin adjuvant), HerpV alone, QS-21 alone and placebo. The vaccine was well tolerated and safe, and adverse events were similar between HerpV and HerpV + QS-21 groups.
Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) vaccines in healthy women aged 18-45 years. Hum Vaccin 2009;5(10):705-719.
The authors compared the immune response and safety of Cervarix®, a monophosphoryl lipid A-containing- HPV vaccine with Gardasil®, an aluminum-adjuvanted HPV vaccine in healthy adult women. They found that both vaccines were well tolerated, though Cervarixwas associated with a higher rate of injection site reactions, fatigue and myalgia, which were transient and resolved spontaneously without sequelae.
Verstraeten T, Descamps D, David MP, Zahaf T, Hardt K, et al. Analysis of adverse events of potential autoimmune aetiology in a large integrated database of AS04 adjuvanted vaccines. Vaccine 2008;26:6630-6638.
The authors assessed the safety of a monophosphoryl lipid A (MPL) containing adjuvant (AS04) in several vaccines (HPV 16/18, HBV, and phase 3 HSV). More than 68,000 patients were evaluated, including more than 39,000 who received HPV-16/18. The authors found a low rate of autoimmune disorders, without evidence of an increased risk associated with ASO4-adjuvanted vaccines versus non-adjuvanted vaccines.
Tong N, Beran J, Kee S, Miguel J, Sanchez C, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int 2005;68:2298-2303.
The authors compared the immunogenicity and safety of an AS04-adjuvanted hepatitis B vaccine with a non-adjuvanted hepatitis B vaccine and found the AS04-adjuvanted vaccine produced a more rapid onset and greater overall level of protection, necessitating fewer booster doses. The adjuvanted vaccine was associated with more injection site reactions, but the rates of serious adverse events were similar between groups, with all events determined to be unrelated to vaccination.
CpG
Hyer R, McGuire DK, Xing B, Jackson S, Janssen R. Safety of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant in adults. Vaccine 2018;36:2604-2611.
The authors describe the safety aspects of three trials comparing a CpG-ODN adjuvanted hepatitis B vaccine with an aluminum-adjuvanted hepatitis B vaccine in more than 13,000 adult subjects. Vaccine–associated adverse events were limited to mild to moderate local and systemic post-injection reactions. The authors found no differences in immune-mediated adverse events between the two groups.
Cooper CL, Davis HL, Morris ML, Efler SM, Kreig AM, et al. Safety and immunogenicity of CPG 7909 injection as an adjuvant to Fluarix influenza vaccine. Vaccine 2004;22:3136-3143.
CPG 7909, a CpG oligodeoxynucleotide (ODN), was tested for safety, tolerability and its ability to augment the immune response of a trivalent influenza vaccine. The authors compared Fluarix® (full or 1/10th dose) plus CPG 7909 to Fluarix® (full or 1/10th dose) plus saline and found all vaccines were generally well tolerated.
Stabilizers in vaccines
Gadzinowski J, Tansey SP, Wysocki J, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine manufactured with and without polysorbate 80 given to healthy infants at 2, 3, 4, and 12 months of age. Pediatr Infect Dis J 2015;34:180-185.
The authors investigated the safety and immunogenicity of PCV-13 manufactured with or without polysorbate 80 (P80) in infants at 2, 3, 4 and 12 months of age. Immunogenicity and safety was similar for both formulations.
Preservatives in vaccines
For a list of studies related to thimerosal (mercury) in vaccines, visit the thimerosal page.
Reviewed by Paul A. Offit, MD, on Aug. 21, 2025
