Newborn and Infant Chronic Lung Disease Program Awarded Prestigious CHOP Frontier Grant
Published on
Neonatology UpdatePublished on
Neonatology UpdateIn its 7 years of existence, the Newborn and Infant Chronic Lung Disease (NeoCLD) Program located within Children’s Hospital of Philadelphia’s Harriet and Ronald Lassin Newborn/Infant Intensive Care Unit (N/IICU) has treated more than 350 infants with severe chronic lung disease (CLD), also called bronchopulmonary dysplasia (BPD). It has grown into an extremely well organized and highly effective multidisciplinary program that provides comprehensive yet individualized care for infants with the most severe form of CLD.
Although many of our patients require long-term ventilation support and prolonged pulmonary hypertension treatment, most of the little graduates from our program are able to achieve long-term stability with continued improvement in cardiopulmonary function over time. At our first CLD patient reunion last October, we witnessed how well these patients thrive at home, which is the best testimony to how important a comprehensive program like ours is to the long-term survival of these most vulnerable patients.
In addition to providing excellent medical care, our NeoCLD team is dedicated to expanding the knowledge base on the disease pathophysiology and management of this condition, and exploring new and advanced treatment options for these patients. Our team members have been actively conducting both retrospective and prospective clinical research that has resulted in at least 25 abstracts presented at national/regional conferences and 20 CLD-related manuscripts published in peer-reviewed journals in the past 5 years.
This combination of extraordinary clinical care, strong clinical research capability and clinical innovation enabled our team to receive a highly competitive CHOP Frontier Program grant for our clinical research activities. We design focused clinical studies with an aim to answer specific questions related to the diagnosis and management issues of infant CLD. Some examples of our recent and ongoing projects are:
With support from the Frontier Program, our team just launched the Fluid Filled Lung Oxygenation Assistance Trial (FFLOAT), a randomized controlled trial to evaluate the safety of Perfluorooctyl Bromide Partial Liquid Ventilation (PFOB-PLV) in infants with severe CLD. Participating infants will be randomized (3:1) to either the PFOB group or a routine care group for 5 days in Part 1 of the study, and, if safety data allows, for up to 10 days in Part 2. This study has been carefully reviewed by the Food and Drug Administration, and approved as an Investigational New Drug for the study of liquid ventilation in neonates.
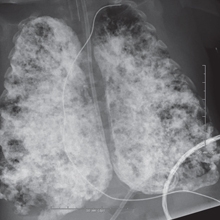 Chest X-ray of an infant with severe CLD after initial filling of 10 ml/kg of PFOB. Note the severe lung disease with un-unified ventilation and multiple areas of filling defects.
This study is the result of a 25-year effort by William Fox, MD, Attending Neonatologist and Medical Director of CHOP’s Infant Breathing Disorder Center, to bring liquid ventilation back as a treatment for infants. Initial liquid ventilation trials were halted in 1996 due to poorly designed adult trials, but the initial infant trials showed promising results. Dr. Fox and his team treated 17 infants between 1992 and 1996, representing the largest patient series studying PFOB-PLV in neonates. They saw no significant adverse events, as documented for adult subjects, and all patients had improvement in lung function and oxygenation. We aim to establish the safety of PFOB-PLV in infants with severe CLD in our initial trial and expand to efficacy studies once safety is established. This potentially could be the first therapy able to change the clinical course of infants with severe CLD.
Chest X-ray of an infant with severe CLD after initial filling of 10 ml/kg of PFOB. Note the severe lung disease with un-unified ventilation and multiple areas of filling defects.
This study is the result of a 25-year effort by William Fox, MD, Attending Neonatologist and Medical Director of CHOP’s Infant Breathing Disorder Center, to bring liquid ventilation back as a treatment for infants. Initial liquid ventilation trials were halted in 1996 due to poorly designed adult trials, but the initial infant trials showed promising results. Dr. Fox and his team treated 17 infants between 1992 and 1996, representing the largest patient series studying PFOB-PLV in neonates. They saw no significant adverse events, as documented for adult subjects, and all patients had improvement in lung function and oxygenation. We aim to establish the safety of PFOB-PLV in infants with severe CLD in our initial trial and expand to efficacy studies once safety is established. This potentially could be the first therapy able to change the clinical course of infants with severe CLD.
The goals of our NeoCLD Program are to provide exceptional clinical care to promote long-term survival with less disability for infants with severe CLD and also advance current knowledge in the management of these patients. We welcome referrals of infants very sick with CLD for comprehensive clinical management and clinical studies
Contributed by: Huayan Zhang, MD
Categories: Division of Neonatology, Neonatology Update